Coordination Compounds Class 12 Notes Pdf
Falco 3 25th anniversary edition youtube. F3, or Falco 3, sounds as good as in Nineteen Eighty-Five. It starts out with 10 tracks from the original album and ends with 6 additional tracks. Which, means.
CBSE Class 12 Chemistry Questions and basic easy to learn concepts/ notes Database for chapter Solid State. Based on CBSE and CCE guidelines. The students should practice these Questions to gain perfection which will help him to get more marks in CBSE examination. Based on CBSE and CCE guidelines. The preparation should be in a manner which helps the students to concentrate more in areas which carry more marks. Please refer to more CBSE Class XII sample papers, question papers, HOTs etc in other links.UNIT 9: C O - ORD IN A TI ON C OMP O UN DThe compounds which contain dative bonds between metal atom and surrounding species is called co-ordination compounds. The branch of inorganic chemistry which deals with hte study of preparation properties of coordination compound is called co-ordination chemistry.K 4 Fe(CN) 6 , CuNH 3 ) 4 SO 4P OI N TS TO REM EM BE R:1.
C oord ina ti on c omp ou ndsC o ord i nat ion co m pou n ds a re co m pou n ds in w h ich a ce nt ral me tal atom or ion is li n ked to anu m b er of ions or ne ut ral mo le c u les by co ord i na te bonds or w h ich con ta in co m p lex io n s. Exa mp l e s- K 4 Fe ( C N) 6 ; C u ( NH 3 ) 4 SO 4; N i( C O)42. T he ma in p o stu la tes of Werne r ’s the o ry of c oord ina ti on c om p ou ndsi ) In co ord i nat ion co m p o un ds meta ls s how t wo t yp es of li n ka g es or va le n cie s- P r i ma ry a nd Se conda r y.ii ) T he p r i ma ry va le n cies a re ion i sa ble a nd a re sat i s f ied by ne gat ive ion s.iii ) T he se con da ry va le n cies a re no n- ion i sa ble a nd a re sat i s fied by ne ut ral mo le c u les or ne gat ive io n s. T he se conda ry va le n ce is e q ual to t he C.N a nd is fi xed for a meta l.i v) T he ions or g ro u ps bou nd by se conda ry li n k a ges to t he metal have c ha ra cte r i st ic s pat ial a rr a n g e m e n ts c o rr e sp o n d i n g to d iff e r e n t c o or d i n a t i on n o s.3. Dif feren ce be tween a d ou b le sa lt and a c omp lexB oth d o u ble sa lts as we ll as co m p l e xes a re fo r med by t he co m bi nat ion of t wo or mo re sta ble co m pou n ds in sto ic h io met r ic rat io.
Ho weve r, d o u ble sa lts s u ch as ca r na llite, K C l. M gCl2.6 H 2 O, Mohr ‘s sa lt, Fe S O 4.
( NH 4 ) 2 S O 4.6 H 2 O, pot a sh a l u m, K A l( S O 4 ) 2.12 H 2 O, et c. D is so c iate i nto s i m p le ions co m p lete ly w h en d i sso lved in wate r.
RyKEY CONCEPTSMolecular / Addition Compound:Molecular / Addition compounds are formed when stoichiometric amounts of two or more simple compounds join together. Molecular / Addition compounds are of two types.Double salts: Those which retain their identity in solutions are called double salts. For example.KCl MgCl 2 6H 2O KCl.MgCl 2. 6H 2OcarnalliteK 2SO 4 Al 2(SO 4) 3 24 H 2O K 2SO 4.Al 2(SO 4 ) 3.24H 2Opotash alumComplex compounds: Those which loose their identity in solution (complexes). For example.CuSO 4 4 NH 3 CuSO 4.4 NH 3 or Cu(NH 3) 4SO 4tetrammine copper (II) sulphateFe(CN 2) 4 KCN Fe (CN 2). 4KCN or K 4Fe(CN) 6potassium ferryocyanideWhen crystals of carnallite are dissolved in water, the solution shows properties of K, Mg 2 and Cl - ions. In a similar way, a solution of potassium alum shows the properties of K, Al 3 and SO 4 2- ions.
Coordination Compounds Class 12 Notes Pdf Online
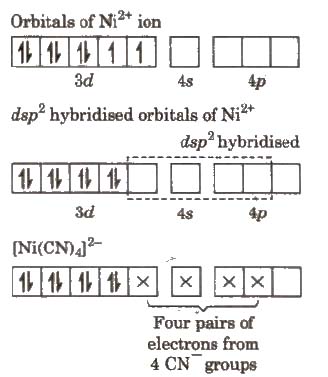
These are both examples of double salts which exist only in the crystalline state. When the other two examples of coordination compounds are dissolved they do not form simple ions, Cu 2 / Fe 2 and CN -, but instead their complex ions are formed.Representation of Complex Ion:whenM = Central Metal atom /ion (usually of d-block)L = Ligandx = No. Of ligands= charge on coordinationOutside region apart from coordination sphere is called ionisation sphere.1. Central metal atom/ion: Central ion acts as an acceptor (Lewis acid) and has to accommodate electron pairs donated by the donor atom of the ligand, it must have empty orbitals. This explains why the transition metals having empty d-orbitals form co-ordination compounds readily. Thus, in complexes Ni(NH 3) 6 2 and Fe(CN) 6 3-, Ni 2 and Fe 3 respectively are the central metal ions.2.
Ligands: Species which are directly linked with the central metal atom/ ion in a complex ion are called ligands. Coordination Compounds - Class 12, Chemistry Notes notes for Class 12 is made by best teachers who have written some of the best books ofClass 12. It has gotten 34229 views and also has 4.5 rating. You can download Free Coordination Compounds - Class 12, Chemistry Notes pdf from EduRev byusing search above. You can also find Coordination Compounds - Class 12, Chemistry Notes ppt and other Class 12 slides as well.
If you want Coordination Compounds - Class 12, Chemistry NotesTests & Videos, you can search for the same too. Class 12 Coordination Compounds - Class 12, Chemistry Notes Summary and Exercise are very important forperfect preparation. You can see some Coordination Compounds - Class 12, Chemistry Notes sample questions with examples at the bottom of this page. CompleteCoordination Compounds - Class 12, Chemistry Notes chapter (including extra questions, long questions, short questions, mcq) can be found on EduRev, you can checkout Class 12 lecture & lessons summary in the same course for Class 12 Syllabus. EduRev is like a wikipediajust for education and the Coordination Compounds - Class 12, Chemistry Notes images and diagram are even better than Byjus! Do check out the sample questionsof Coordination Compounds - Class 12, Chemistry Notes for Class 12, the answers and examples explain the meaning of chapter in the best manner.

This isyour solution of Coordination Compounds - Class 12, Chemistry Notes search giving you solved answers for the same. To Study Coordination Compounds - Class 12, Chemistry Notes for Class 12this is your one stop solution.